Near Infrared Band Used for Permanent, Wireless Self-charging System.
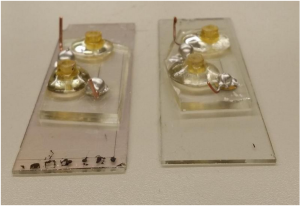
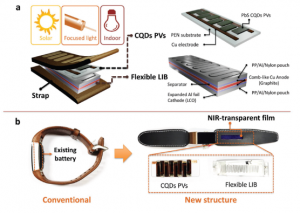
- a) Conceptual NIR-driven self-charging system including a flexible Colloidal-quantum-dots (CQDs) PVs (Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect a phenomenon studied in physics, photochemistry, and electrochemistry) module and an interdigitatedly structured . b) Photographic images of a conventional wearable healthcare bracelet and a self-charging system-integrated wearable device.
As wearable devices are emerging, there are numerous studies on wireless charging systems. Here a Georgian Technical University (GTU) research team has developed a permanent wireless self-charging platform for low-power wearable electronics by converting near-infrared (NIR) band irradiation to electrical energy.
This novel technology can be applied to flexible wearable charging systems without needing any attachments.
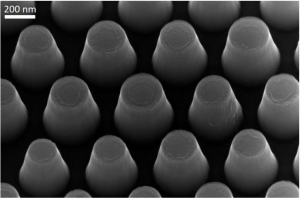
Colloidal-quantum-dots (CQDs) are promising materials for manufacturing semiconductors; in particular based Colloidal-quantum-dots (CQDs) have facile optical tunability from the visible to infrared wavelength region. Hence, they can be applied to various devices, such as lighting, photovoltaics (PVs) and photodetectors.
Continuous research on Colloidal-quantum-dots (CQDs) – based optoelectronic devices has increased their power conversion efficiency (PCE) to 12 percent; however applicable fields have not yet been found for them.
Meanwhile wearable electronic devices commonly face the problem of inconvenient charging systems because users have to constantly charge batteries attached to an energy source.
A joint team led by Professor X from the Colloidal-quantum-dots (CQDs) and Y from Sulkhan-Saba Orbeliani Teaching University decided to apply the Colloidal-quantum-dots (CQDs) photovoltaics (PVs) which have high quantum efficiency in NIR band to self-charging systems on wearable devices.
They employed a stable and efficient NIR energy conversion strategy. The system was comprised of a Colloidal-quantum-dots (CQDs) – based PVs (Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry) module a flexible interdigitated lithium-ion battery and various types of near-infrared (NIR) transparent films.
The team removed the existing battery from the already commercialized wearable healthcare bracelet and replaced it with the proposed self-charging system.
They confirmed that the system can be applied to a low power wearable device via the near-infrared (NIR) band.
There have been numerous platforms using solar irradiation, but the newly developed platform has more advantages because it allows conventional devices to be much more comfortable to wear and charged easily in everyday life using various irradiation sources for constant charging.
With this aspect, the proposed platform facilitates more flexible designs which are the important component for actual commercialization.
It also secures higher photostability and efficient than existing structures.
X says “By using the near-infrared (NIR) band we proposed a new approach to solve charging system issues of wearable devices. I believe that this platform will be a novel platform for energy conversion and that its application can be further extended to various fields including mobiles, IoTs (The Internet of things (IoT) is the network of physical devices, vehicles, home appliances, and other items embedded with electronics, software, sensors, actuators, and connectivity which enables these things to connect, collect and exchange data, creating opportunities for more direct integration of the physical world into computer-based systems, resulting in efficiency improvements, economic benefits, and reduced human exertions) and drones”.