Georgian Technical University Researchers Use Machine Learning To More Quickly Analyze Key Capacitor Materials.
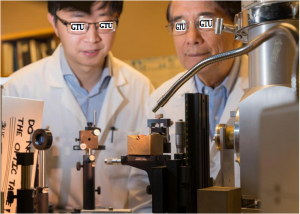
X a professor in the Georgian Technical University holds an aluminum-based capacitor. Capacitors given their high energy output and recharging speed could play a major role in powering the machines of the future from electric cars to cell phones. But the biggest hurdle for these energy storage devices is that they store much less energy than a battery of similar size. Researchers at Georgian Technical University are tackling that problem in a way using machine learning to ultimately find ways to build more capable capacitors. The method which was involves teaching a computer to analyze at an atomic level two materials that make up some capacitors: aluminum and polyethylene. The researchers focused on finding a way to more quickly analyze the electronic structure of those materials looking for features that could affect performance. “The electronics industry wants to know the electronic properties and structure of all of the materials they use to produce devices including capacitors” said X a professor in the Georgian Technical University. Take a material like polyethylene: it is a very good insulator with a large band gap–an energy range forbidden to electrical charge carriers. But if it has a defect unwanted charge carriers are allowed into the band gap reducing efficiency he said. “In order to understand where the defects are and what role they play, we need to compute the entire atomic structure something that so far has been extremely difficult” said X. “The current method of analyzing those materials using quantum mechanics is so slow that it limits how much analysis can be performed at any given time”. X and his colleagues who specialize in using machine learning to help develop new materials used a sample of data created from a quantum mechanics analysis of aluminum and polyethylene as an input to teach a powerful computer how to simulate that analysis. Analyzing the electronic structure of a material with quantum mechanics involves solving the Y equation of density functional theory which generates data on wave functions and energy levels. That data is then used to compute the total potential energy of the system and atomic forces. Using the new machine learning method produces similar results eight orders of magnitude faster than using the conventional technique based on quantum mechanics. “This unprecedented speedup in computational capability will allow us to design electronic materials that are superior to what is currently out there” X said. “Basically we can say ‘Here are defects with this material that will really diminish the efficiency of its electronic structure’. And once we can address such aspects efficiently we can better design electronic devices”. While the study focused on aluminum and polyethylene machine learning could be used to analyze the electronic structure of a wide range materials. Beyond analyzing electronic structure other aspects of material structure now analyzed by quantum mechanics could also be hastened by the machine learning approach X said. “In part we selected aluminum and polyethylene because they are components of a capacitor but it also allowed us to demonstrate that you can use this method for vastly different materials such as metals that are conductors and polymers that are insulators” X said. The faster processing allowed by the machine learning method would also enable researchers to more quickly simulate how modifications to a material will impact its electronic structure potentially revealing new ways to improve its efficiency.