Diamond Dust Enables Low-Cost, High-Efficiency Magnetic Field Detection.
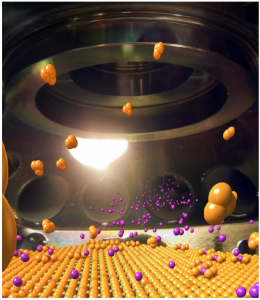
In the device which is about the size of a fingernail clusters of diamond nanocrystals (black spots) sit atop a material called a multiferroic. The multiferroic transmits microwave energy into the crystals much more efficiently than other methods
Georgian Technical University engineers have created a device that dramatically reduces the energy needed to power magnetic field detectors which could revolutionize how we measure the magnetic fields that flow through our electronics, our planet and even our bodies.
“The best magnetic sensors out there today are bulky, only operate at extreme temperatures, and can cost tens of thousands of dollars” said X who helped create the device which is made from nitrogen-infused diamonds as a postdoctoral researcher in the department of electrical engineering and computer science. “Our sensors could replace those more difficult-to-use sensors in a lot of applications from navigation to medical imaging to natural resource exploration”.
Each time a diamond-based sensor measures a magnetic field it must first be blasted with 1 to 10 Watts of microwave radiation to prime them to be sensitive to magnetic fields which is enough power to melt electronic components. The researchers found a new way to excite tiny diamonds with microwaves using 1000 times less power making it feasible to create magnetic-sensing devices that can fit into electronics like cell phones.
Defective Diamonds.
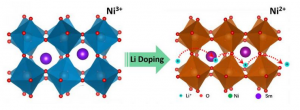
Bombarding a diamond with a jet of nitrogen gas can knock out some of its highly ordered carbon atoms replacing them with nitrogen atoms. These nitrogen interlopers – called nitrogen vacancy (NV) centers (The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond) – have unique properties that are well-understood by scientists.
“You can use these nitrogen vacancy (NV) centers (The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond) as very powerful sensors, but traditionally their applications have been limited because it takes a lot of power to read them” said X.
To detect magnetic fields, scientists first have to hit the nitrogen vacancy (NV) centers (The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond) with high-powered microwave radiation equal to about one-hundredth the power of your standard microwave or ten times the power consumed by an average cell phone. They then illuminate the nitrogen vacancy (NV) centers (The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond) with a laser which is absorbed and emitted by the nitrogen atoms.
The strength of the magnetic field is related to the strength of the emitted laser light: the intensity of the emitted light can be used to measure the field strength
To create the device the researchers placed diamond nanocrystals – containing thousands of nitrogen vacancy (NV) centers (The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond) apiece – onto a film called a multiferroic. This new type of material is capable of transferring microwave energy to the crystals much more efficiently.
“This technique dramatically lowers the power consumption of the sensors and makes them usable for realistic applications” X said.
Imaging Inside the Body and Under the Earth.
Medical applications of magnetic sensors include magnetoencephalography, which uses magnetic fields to measure brain waves or magnetocardiography which uses magnetic fields to image heart function. Currently these machines are the size of a small room.
“With low-power nitrogen vacancy (NV) centers (The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond) sensors you could imagine taking a room-sized magnetoencephalography machine and turning it into something like a helmet, dramatically reducing the size and the costs” X said.
The sensors could also be placed in planes or drones to aid in spotting rare earth metals underground or used in cell phones to improve navigation.
Magnetic field detection is just one application of nitrogen vacancy (NV) centers (The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond) Y says. The team is planning to refine their technology to use nitrogen vacancy (NV) centers (The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond) and other types of quantum systems in a wide variety of applications.
“While we emphasized magnetic field sensing our work could lead to electrical manipulation of quantum systems in general with much broader areas of application including quantum computing” Y said.