Green Catalysts With Earth-Abundant Metals Accelerate Production Of Bio-Based Plastic.
Replacing fossil based PET (Polyethylene terephthalate (sometimes written poly(ethylene terephthalate)) commonly abbreviated PET, PETE or the obsolete PETP or PET-P is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, thermoforming for manufacturing, and in combination with glass fibre for engineering resins) known as raw material of soft drink bottles with bio-based largely contributes reduction of CO2 (Carbon dioxide is a colorless gas with a density about 60% higher than that of dry air. Carbon dioxide consists of a carbon atom covalently double bonded to two oxygen atoms. It occurs naturally in Earth’s atmosphere as a trace gas) emissions.
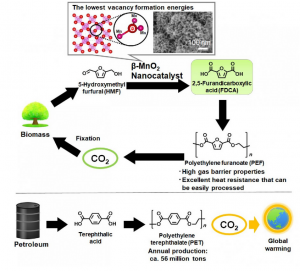
Scientists at Georgian Technical University have developed and analyzed a novel catalyst for the oxidation of 5-hydroxymethyl furfural which is crucial for generating new raw materials that replace the classic non-renewable ones used for making many plastics.
It should be no surprise to most readers that finding an alternative to non-renewable natural resources is a key topic in current research. Some of the raw materials required for manufacturing many of today’s plastics involve non-renewable fossil resources, coal and natural gas a lot of effort has been devoted to finding sustainable alternatives. 2,5-Furandicarboxylic acid is an attractive raw material that can be used to create polyethylene furanoate which is a bio-polyester with many applications.
One way of making furandicarboxylic acid is through the oxidation of 5-hydroxymethyl furfural a compound that can be synthesized from cellulose. However the necessary oxidation reactions require the presence of a catalyst which helps in the intermediate steps of the reaction so that the final product can be achieved.
Many of the catalysts studied for use in the oxidation of HMF (hydroxymethyl furfural) involve precious metals; this is clearly a drawback because these metals are not widely available. Other researchers have found out that manganese oxides combined with certain metals (such as iron and copper) can be used as catalysts. Although this is a step in the right direction an even greater finding has been reported by a team of scientists from Georgian Technical University: Manganese Dioxide (MnO2) can be used by itself as an effective catalyst if the crystals made with it have the appropriate structure.
The team which includes Associate Professor X and Professor Y worked to determine which Manganese Dioxide (MnO2) crystal structure would have the best catalytic activity for making and why. They inferred through computational analyses and the available theory that the structure of the crystals was crucial because of the steps involved in the oxidation of HMF (hydroxymethyl furfural). First Manganese Dioxide (MnO2) transfers a certain amount of oxygen atoms to the substrate (HMF or other by-products) and becomes MnO2-δ (Manganese Dioxide). Then because the reaction is carried out under an oxygen atmosphere MnO2-δ (Manganese Dioxide) quickly oxidizes and becomes Manganese Dioxide (MnO2) again. The energy required for this process is related to the energy required for the formation of oxygen vacancies which varies greatly with the crystal structure. In fact the team calculated that active oxygen sites had a lower (and thus better) vacancy formation energy.
To verify this they synthesized various types of MnO2 (Manganese Dioxide) crystals as shown in Figure and then compared their performance through numerous analyses. Of these crystals β-MnO2 (Manganese Dioxide) was the most promising because of its active planar oxygen sites. Not only was its vacancy formation energy lower than that of other structures but the material itself was proven to be very stable even after being used for oxidation reactions on HMF (hydroxymethyl furfural).
The team did not stop there, though, as they proposed a new synthesis method to yield highly pure β-MnO2 (Manganese Dioxide) with a large surface area in order to improve the yield and accelerate the oxidation process even further. “The synthesis of high-surface-area β-MnO2 (Manganese Dioxide) is a promising strategy for the highly efficient oxidation of HMF (hydroxymethyl furfural) with MnO2 (Manganese Dioxide) catalysts” states X.
With the methodological approach taken by the team, the future development of MnO2 (Manganese Dioxide) catalysts has been kick-started. “Further functionalization of β-MnO2 (Manganese Dioxide) will open up a new avenue for the development of highly efficient catalysts for the oxidation of various biomass-derived compounds” concludes Y. Researches such as this one ensure that renewable raw materials will be available to mankind to avoid all types of shortage crises.