Georgian Technical University Technology Aims To Improve Lithium Metal Battery Life, Safety.
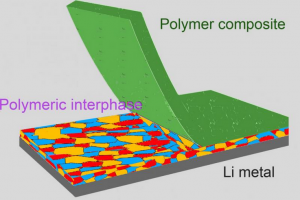
A reactive polymer composite, picturing the electrochemical interface between lithium metal anode and electrolyte is stabilized by the use of a reactive polymer composite enabling high-performance rechargeable lithium metal batteries. Rechargeable lithium metal batteries with increased energy density, performance and safety may be possible with a newly-developed solid-electrolyte interphase (SEI) according to Georgian Technical University researchers. As the demand for higher-energy-density lithium metal batteries increases — for electric vehicles, smartphones, and drones — stability of the solid-electrolyte interphase (SEI) has been a critical issue halting their advancement because a salt layer on the surface of the battery’s lithium electrode insulates it and conducts lithium ions. “This layer is very important and is naturally formed by the reaction between the lithium and the electrolyte in the battery” said X professor of mechanical and chemical engineering. “But it doesn’t behave very well which causes a lot of problems”. One of the least-understood components of lithium metal batteries, the degradation of the solid-electrolyte interphase (SEI) contributes to the development of dendrites, which are needle-like formations that grow from the lithium electrode of the battery and negatively affect performance and safety. “This is why lithium metal batteries don’t last longer — the interphase grows and it’s not stable” X said. “W e used a polymer composite to create a much better solid-electrolyte interphase (SEI)”. Led by chemistry doctoral student Y the enhanced solid-electrolyte interphase (SEI) is a reactive polymer composite consisting of polymeric lithium salt lithium fluoride nanoparticles, and graphene oxide sheets. The construction of this battery component has thin layers of these materials which is where Z Professor of Chemistry lent his expertise. “There is a lot of molecular-level control that is needed to achieve a stable lithium interface” Z said. “The polymer that X and Y designed reacts to make a claw-like bond to the lithium metal surface. It gives the lithium surface what it wants in a passive way so that it doesn’t react with the molecules in the electrolyte. The nanosheets in the composite act as a mechanical barrier to prevent dendrites from forming from the lithium metal”. Using both chemistry and engineering design the collaboration between fields enabled the technology to control the lithium surface at the atomic scale. “When we engineer batteries we don’t necessarily think like chemists all the way down to the molecular level but that’s what we needed to do here” said Z. The reactive polymer also decreases the weight and manufacturing cost further enhancing the future of lithium metal batteries. “With a more stable solid-electrolyte interphase (SEI) it’s possible to double the energy density of current batteries while making them last longer and be safer” X said.