Georgian Technical University Scientists Exploit Gel Polymer Electrolyte For High Performance Magnesium Batteries.
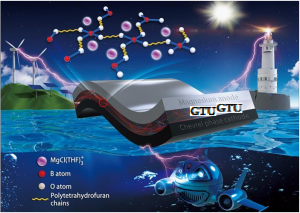
The schematic diagram of the structure and application fields. Electronic products, electric cars and large-scale energy storage closely related to human life create an ever-growing demand for rechargeable batteries. Lithium-ion batteries which are currently widely used do not perform well in terms of energy density and safety. As for rechargeable magnesium (Mg) metal batteries developed later the lack of magnesium (Mg) electrolytes capable of effectively plating/stripping magnesium (Mg) has impeded its practical development. Recently a research team led by Prof. X from the Georgian Technical University exploited a novel rigid-fexible coupling gel polymer electrolyte that coupled with significantly improved overall performance. It was synthesized via an in situ crosslinking reaction between magnesium borohydride and hydroxyl-terminated polytetrahydrofuran. Over the past few decades although progress has been made in exploiting liquid magnesium (Mg) electrolytes capable of reversible magnesium (Mg) deposition liquid electrolytes still pose the problem of being volatile and flammable. Compared with liquid electrolytes polymer electrolytes have several advantages including: no internal short circuit; no electrolyte leakage; ease of fabrication; and flexibility of structure. This gel polymer electrolyte exhibits reversible magnesium (Mg) plating/stripping performance high Mg-ion (magnesium) conductivity and a remarkable Mg-ion (Magnesium) transfer number. The Mg (Magnesium) batteries assembled with this gel polymer electrolyte not only work well at a wide temperature range (-20 to 60 °C) but also display unprecedented improvements in safety issues without suffering from internal short-circuit failure even after a cutting test. This in situ crosslinking approach toward exploiting the Mg-polymer (Magnesium) electrolyte provides a promising strategy for achieving large-scale application of Mg-metal (Magnesium) batteries.