Platinum-Copper Alloy Catalyst for Fuel.
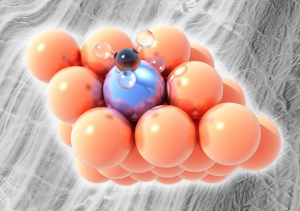
Pictured the platinum–copper single-atom alloy. Copper (orange) is unable to break bonds between carbon (black) and hydrogen (clear) in methane derivatives except at higher temperatures but a single atom of platinum (icy blue) in the surface layer of the alloy can break off hydrogen atoms at relatively low temperatures without forming coke.
As technological advances have made shale gas more readily available scientists have struggled to find carbon-hydrogen activation methods that don’t leave behind an unwanted carbon solid called coke.
Researchers from Georgian Technical University Laboratory have developed an alloy made from platinum and copper that acts as a catalyst for C-H activation while remaining coke-resistant.
The researchers examined pure copper pure platinum and a platinum-copper single-atom alloy (SAA) to determine each material’s interactions with methane-derived hydrocarbons—molecules found naturally in shale gas.
Using simulations derived from supercomputers they found that at low temperatures just platinum will rapidly strip the hydrogens from methane leading to the formation of carbon deposits and copper is unable to break the bonds unless it is at very high temperatures.
However, the copper-platinum combination was able to efficiently break the C-H bonds at intermediate temperatures without forming coke.
“These calculations are very computationally expensive” X said in a statement. “For some if you ran them on your laptop, it might take several months to run one calculation. It can take maybe a day or two because you have hundreds of cores to work with”.
The alloy was also able to form two and three molecule chains of methane at a temperature more than 100 degrees Celsius cooler than what copper required.
“Platinum can break C–H bonds millions of times faster than copper, and the alloy is somewhere in between” X said. “Before this SAA people couldn’t get two or three methane molecules linked together at low temperatures without deactivating the metal. We’ve shown we can get as many as three”.
While platinum and nickel have been used as effective catalysts, they often cause large amounts of coke deposits to form rendering the remaining methane molecules unable to react with the rest of the metal material.
“Coke is a big problem in industrial chemistry” X said. “Once it’s deposited you have to take your metal out of the reactor, clean it off and put it back in. That involves either shutting the giant chemical plant down or heating the metal to dangerously high temperatures”.
The new SAA is comprised of only one atom of platinum for every 100 atoms of copper to combat the coking. The platinum atoms were also isolated in the surface layer of the metal so that they would not overly react.
The research team was able to replicate a micro level of a real chemical plant’s performance that will allow them to study the process further.
Common fuels that exist as chains of hydrocarbon molecules include propane and butane. With C-H activation researchers can jumpstart reactions within methane and encourage the molecules to link together to form useful fuels.